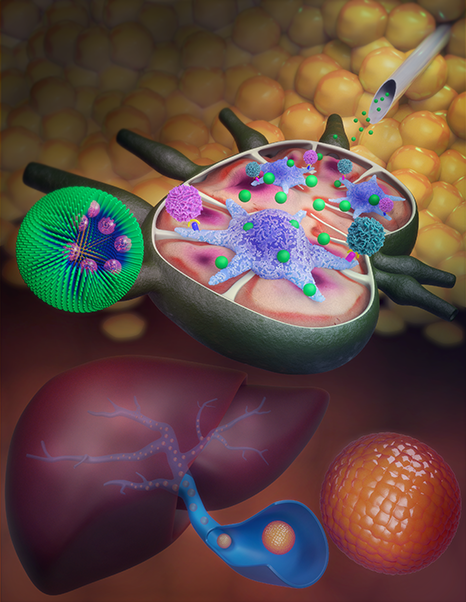
Northwestern University researchers developed a nanoparticle delivery system for a common immunosuppressive drug that increases the potential of pancreatic islet cell transplantation as a viable long-term treatment for type 1 diabetes. The technology aims to ensure that the drug acts on the antigen-presenting cells of the immune system and not on T-cells. This results in more selective immunosuppression with fewer side effects and better long-term viability for transplanted islets that are typically targeted and destroyed by the immune system. Researchers hope the technology could pave the way for islet transplantation as a viable treatment, but also increase the potential for transplantation of other tissues and organs.
Currently, type I diabetes requires regular blood glucose measurements and insulin injections. Even with more advanced techniques like insulin pumps, such patients are still a lifelong burden. Pancreatic islet transplantation could change this by providing long-term control of blood sugar levels, but the technique is still hampered by immune rejection of the transplanted tissue.
Conventional immunosuppressive drugs such as rapamycin currently do not adequately protect the islets, at least not at safe doses. The side effects of such drugs can be difficult to bear, including reduced immune protection against infections like COVID-19. “To avoid the broad effects of rapamycin during treatment, the drug is typically given at low doses and through specific routes of administration, primarily orally,” said Evan Scott, a researcher involved in the study. “But in the case of a transplant, you have to give enough rapamycin to suppress T cells systemically, which can have significant side effects like hair loss, mouth sores, and an overall weakened immune system.”

To address this, the Northwestern University researchers used nanoparticles to target rapamycin specifically to antigen-presenting cells in the immune system, rather than the T-cells it normally affects. This results in a more controlled immunosuppression that appears to balance protection for transplanted pancreatic islets with a reasonable safety profile.
“We wondered if rapamycin could be redesigned to avoid non-specific T-cell suppression and instead stimulate a tolerogenic pathway by delivering the drug to different types of immune cells?” said Scott. “By changing the target cell types, we actually changed the way immunosuppression was achieved.”
So far, researchers have tested the technique on diabetic mice that had received pancreatic islet cell transplants. Notably, the mice showed minimal side effects but did not develop diabetes during the 100-day experiment, suggesting the treatment was working to protect the islets.
Studies in natural nanotechnology: Subcutaneous nanotherapy repurposes the immunosuppressive mechanism of rapamycin to improve the viability of islet allografts
Over: Northwest University